Scaling a European medtech start-up in Asia
A case of surgical robotics innovation (and minimally invasive Pokémon)
This completes a series of three articles about surgical robotics.
The previous two articles were about:
How surgical robotics will change surgery
The surgical robotics adoption problem
TL;DR
One way to increase surgical robot adoption is to altogether re-think how they are designed
Interventional Systems has done just this, and is well on the path to making them more affordable
Scaling a European robot surgery medtech in Asia comes with unique challenges
Knowing how to structure and operate a local joint venture, having local support for regulatory clearance, and keeping simple and clear communication are all crucial to growing in Asia
Arise, Minimally Invasive Magikarp!
Before we get into the guts of the story, I recently read about the curious case of a surgeon playing Pokémon Go whilst preparing for surgery…
Hey, surgeons are a stressed bunch – so why not let out your inner Ash Ketchum during a bit of idle time - gotta catch’em all!

OK, so I can’t imagine you’d want to hear about your surgeon playing video games in between surgeries, but maybe this is a symptom (no pun intended, really) that comes with the stress and intensity of surgery… and perhaps also with operating cumbersome surgical robots.
It’s high time for changes so that robot surgery becomes more accepted by both patients and surgeons worldwide.
Let’s get to know a company adopting a fresh approach, and how the sights they set on Asia are now in motion.
Boom.
Interventional Systems: a different approach
“We want to be able to reach any target, head to toe, with sub-millimetre accuracy, under any form of image guidance, be it CT, MRI, ultrasound, fluoroscopy, you name it”

An upmarket ski resort town is not the first place you would expect to find a company at the forefront of surgical robotics. But that is exactly where an enterprising team of surgical tech entrepreneurs decided to set up shop.
Nested in the leafy green hills of Kitzbühel, Austria, is Interventional Systems (INS for short), a company creating cost-effective, compact and portable, simple-to-use surgical positioning solutions that can be used with any imaging modality.
Dr. Michael Vogele, CEO, co-founded INS with Thomas Pfeifer, who leads the commercial and financial side of the business. The two have known each other since childhood and decided to join forces in 2010 after Michael sold his previous business, Medical Intelligence, to Swedish radiation therapy leader Elekta.
Medical Intelligence created an image-guided robotic patient positioning system called the HexaPOD which gave practitioners six degrees of freedom and sub-millimetre accuracy to perform stereotactic radiation therapy and stereotactic radiosurgery, which is not surgery in the normal sense because it uses 3D images to target high doses of radiation to tumours without any incisions.
For CEO Dr. Michael Vogele, a self-professed adopted Tyrolean (who hasn’t forgotten his Bavarian roots) the idea behind starting Interventional Systems came not from the urge to join the minimally-invasive surgery bandwagon, but from something closer to his background in radiation therapy.
Coming from the stereotactic world where computers and 3D scanning devices are used locate surgical targets, I found the best way to accurately place surgical instruments was to thread a tiny endoscope through a trocar which had 7 degrees of freedom, so that it could be properly angled and rotated.
This was the patient’s radiation exposure is significantly reduced, and you avoid needing to directly insert a surgical needle altogether!
This also happens to be why Michael prefers not to even call INS’s product a robot! 🤖

When it comes to surgical robotics, the image of multiple, bulky, flailing mechanical arms would not be far off how most robot surgery companies thought their products should look. Companies were mostly repurposing pick-and-place industrial robot arms (think the big ones from ABB, Kuka and Mitsubishi) and re-creating them into something to fit the bill for surgery.
As Pedro Costa, INS’s Chief Product Officer who joined in 2016 with a deep background in surgical robotics research explained, whilst INS also started out prototyping with large Mitsubishi industrial robots, they soon realised this wasn’t the right approach:
With large industrial-type robotic systems you have many challenges applying them to surgery. For example, you end up with coupled motion, where one movement forces all the other robot joints to move in tandem, making reachability of the robot arms within a small space very limited…
…we figured that if you use the same tech you will only get incremental improvements, so we had to go back to the drawing board and think of an alternative to “pick-and-place”
INS spent their early years spinning and selling off innovations and IP to major medtech players. Starting with a mini robot for cranial surgery, INS jointly developed a surgical positioning system which was first licensed and eventually sold off to the market leader in cranial neurosurgery.
INS: rethinking the form factor for surgical robots
(Oops, mentioned that in the same breath as INS, sorry Michael!)
Jokes aside
Today, INS’s flagship product is their FDA 510(k)-cleared Micromate™ table-mounted surgical guidance robot which helps surgeons accurately pinpoint where to create the first incision for a trocar to be inserted into the body.
This approach means their robot arm can hold surgical instruments the same way a human holds a pen, which is naturally the method that yields the most manoeuvrability, as opposed to restrictive, vertical manipulation used by pick-and-place type robots we are mostly familiar with.
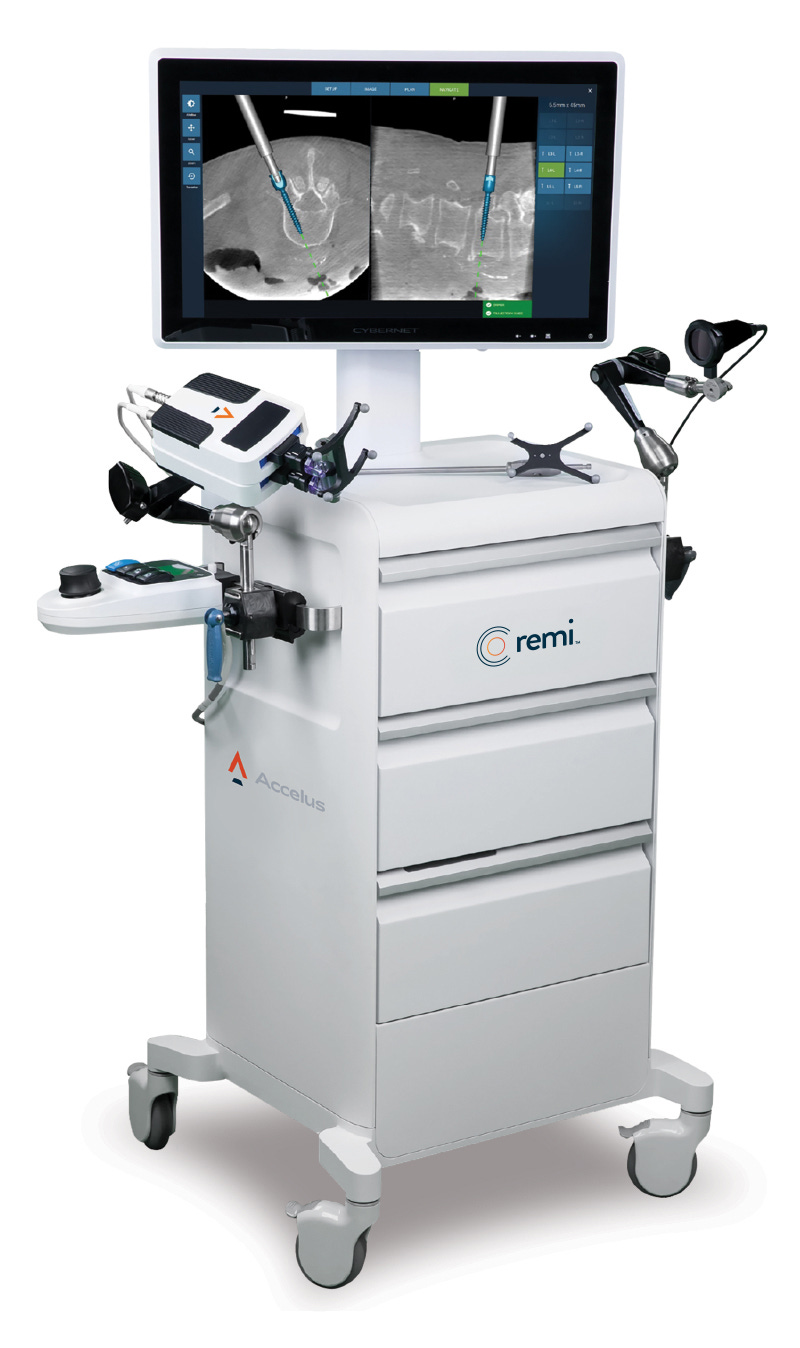
INS has always been globally-minded, and in 2019 they invested in and set up a joint venture with Fusion Robotics in the US, world experts in spinal procedures. The JV has since merged with Integrity Implants to become Accelus, co-engineering the Accelus REMI system in anticipation of market dominance (doesn its design look familiar? 😆).
INS also completed feasibility studies on a robot that fits into the gantry of an MRI machine — something no other surgical guidance robot can do - making real-time MRI possible under strong magnetic fields. Normally the patient would need to be moved and surgery paused to perform MRI — not ideal for anyone if you just wanted to get on with the surgery.
Solving the issue of cost and utilisation
INS took an important step to address the problem of worse-than-desired utilisation of robots in surgery — by rolling out a novel pay-per-month business model.
If we think back to how Intuitive Surgical makes sales, their business model revolves around selling their robots (as capital equipment, often on leases), surgical instruments and services with their robots — and doctors are nudged strongly towards buying their instruments since their pre-integration with the Da Vinci platform offers added connectivity functions. Intuitive is really raking in the money here. But with robots such as Da Vinci’s costing at least a million USD a pop, you start to see why this prohibits wider adoption in the market. Simply put, the ROI for buyers becomes challenging to justify, and means hospital purchasing decisions often need to be approved at the highest levels of the hierarchy.
Instead, INS is bundling a fixed monthly subscription fee covering a batch of consumables, free software upgrades, as well as onboarding support — something no other surgical robot company in the market is able to offer because of their high price tags. Pretty neat. So by capitalising on Micromate’s affordable price tag, INS is bucking this trend of hefty service agreements and charging substantial amounts for consumables.
A Bright Future in Asia
Still a vast and open market for surgical robotics, Asia is seen as green pastures for adoption and expansion. Industry frontrunner Intuitive Surgical made it a strategic priority very early on to expand Da Vinci across the region — in 2020 China even became their second largest market for the number of procedures performed, and presented a far higher utilisation rate for their robots compared to their US home market1.
For INS, Asia has similarly been a strategic priority, front and centre. So it was great to hear from INS’s Chief Product Officer, Pedro Costa, about INS’s foray into China:
“It was very strategic for us to get onto China’s 2030 programme to adopt healthcare technologies from around the world, and also ensure we localised in order to enter this vast market”
INS has worked super hard to get to where they are on the China front, and thanks to supportive shareholders across the board, they have already developed multiple China opportunities. And Pedro is positive on China’s refreshing attitude to medtech innovation:
“China views medtech innovation as an urgent and long-term strategic priority, but also accepts these things take time, and it’s like local teams are looking to speed up the sustainability of tech adoption by “slowing down to speed other things up”. In other words they want to get through all the key learnings to make sure the end result is solid to avoid unnecessary backtracking. It’s a nice paradox.”
Seizing the China opportunity by the horns
2021, in the Chinese Zodiac, was the year of the Ox, and what better way to celebrate than by inking a landmark joint venture deal with Chinese surgical tools and consumables innovator Ningbo Hicren, a deal that pairs INS’s micro-invasive solutions with Hicren’s innovations in implants and surgical tools for spine and sports medicine. Most importantly, INS can now tap into a vast network of local KOLs to ramp up product visibility in China.
So what do Western medtech companies need to look out to scale in a place like China where the installation base of surgical robots is expected to grow 23% per year to 2030? We’ll break some of these down based on Pedro’s experience.
Joint venture
INS positioned itself as the R&D brains of the partnership, keeping key know-how, sourcing and core manufacturing remaining with INS HQ in Europe, tasking the JV with assembling the final product. Locally, non-core manufacturing can be done, and China could eventually even become a second source of full-scale manufacturing in the future once scale is achieved. In the JV world, the old adage is the more you own of the JV, the better pricing power you have. No doubt a useful template for others thinking about Asian expansion.
Clinical trials
INS is completing the final steps of their clinical trials in China, which is usually a 3-year process. This needs the help of a local partner, otherwise things can easily be held up and costs would be harder to control. This arrangement can even help clinical trials to become more focussed and efficient — for example, instead of analysing the effect on the whole abdomen, the Chinese partner can help focus analyses on the impacts on specific organs. In contrast to Europe and the US where INS’s device is considered a smart targeting device, China categorises INS and other surgical robots in a higher risk category, so clinical trials are pretty much unavoidable. The JV really streamlines these initiatives.
Regulatory approvals
In China, the National Medical Products Administration (NMPA, China’s equivalent of FDA in the US) is very strict and prescriptive in terms of approvals. As an example, in the West, doctors use third-party imaging devices to help the robot control the surgical needle. In China, because most local surgical robot products include this feature, NMPA will still require this even if it is not part of the core product offering, simply because other products in the market have these features. In fact, this feature of local regulations means that cross-pollination between different fields is driving a lot of innovation in medtech product development in China.
Communication
Quality management processes differ between Europe/US and China. Teams across the East-West divide are not always familiar with how each other does things. Some companies, such as Hicren, have people with abundant experience working with Western project management styles, and also have a good command of English. Other companies may need to be handheld a bit more. Breaks in conversations often occur where discussions need to be translated between Chinese and English before resuming. So, the best thing is always to over-communicate about what one another is doing in as plain and simple English as possible. Setting clear roles and responsibilities for what the local JV partner and European partner should do is crucial to avoid misunderstandings which could slow things down or even lead to conflicts.
And if people wanted to know what INS’s CPO thought about their progress so far in China:
“A great journey so far, and we have a setup that gives us real creative input and say in terms of how we go ahead in China. It’s been very positive partnering with our JV shareholders.”

Surgical robotics is a technical yet fascinating industry which we are increasingly likely to cross paths with in a healthcare setting during our lives and is helping to bring about a paradigm shift.
With considerable barriers to entry erected by the likes of ISRG and many issues with adoption, its worth remembering the sector is still relatively immature, and the next 5-10 years may look quite different with so many players joining the party. It’s worth noting there are many innovations coming up which will hopefully make our life better, thanks to the growth of the sector.
And for up-and-coming surgical robotics companies in Europe with dreams of Asian expansion, proper research on what local governments are doing and and coordinating carefully with local partners on the ground will be essential.
That’s all for this edition folks. Thanks so much for reading, and stay tuned.
Was this article on scaling a medtech in Asia useful? Help me to improve!
With your feedback, we can improve the letter. Click on a link to vote:
Build your own? 👉 FeedLetter.co
All views contained in this article are my own and do not necessarily represent the views of any other organisation.
None of the above constitutes investment advice in any way.
iSYS Medizintechnik GmbH (Interventional Systems) is a portfolio company of Ascend Capital Partners.
Intuitive Surgical’s Asia opportunity
https://www.fool.com/investing/2021/04/03/in-asia-intuitive-surgicals-growth-story-is-just-b/